Model NO.: infusion set
Logo Printing: With Logo Printing
Medical Device Regulatory Type: Type 3
Medical Devices Reg./Record No.: Gxzz20153661379
Brand: Jmd or OEM
Certificates: CE, ISO or GMP
ISO Certificate: ISO13485, ISO9001
Trademark: JMD or OEM
Transport Package: PE or Blister
Specification: CE, ISO, GMP
Origin: Changzhou, China
HS Code: 90183900
Model NO.: infusion set
Logo Printing: With Logo Printing
Medical Device Regulatory Type: Type 3
Medical Devices Reg./Record No.: Gxzz20153661379
Brand: Jmd or OEM
Certificates: CE, ISO or GMP
ISO Certificate: ISO13485, ISO9001
Trademark: JMD or OEM
Transport Package: PE or Blister
Specification: CE, ISO, GMP
Origin: Changzhou, China
HS Code: 90183900
PRODUCT SPECIFICATION
| Type |
infusion set |
| Material |
PVC |
| Certification |
CE, SGS ,ISO13485 |
| Sterilization |
Ethylene Oxide |
| Quality Guarantee Period |
3 years |
| Package |
PE or Blister |
| Specification |
CE , ISO , GMPÂ |
| Tube material |
PVC/Non- PVCÂ |
| Feature |
Disposable |
| Needle |
with and without needle |
| stype |
Luer Slip or Luer lock |
| Tube length |
1.5m or OEMÂ |
| Components |
Plastic Spike |
| Air vent |
With / without air vent |
| Filter |
with / without filter |



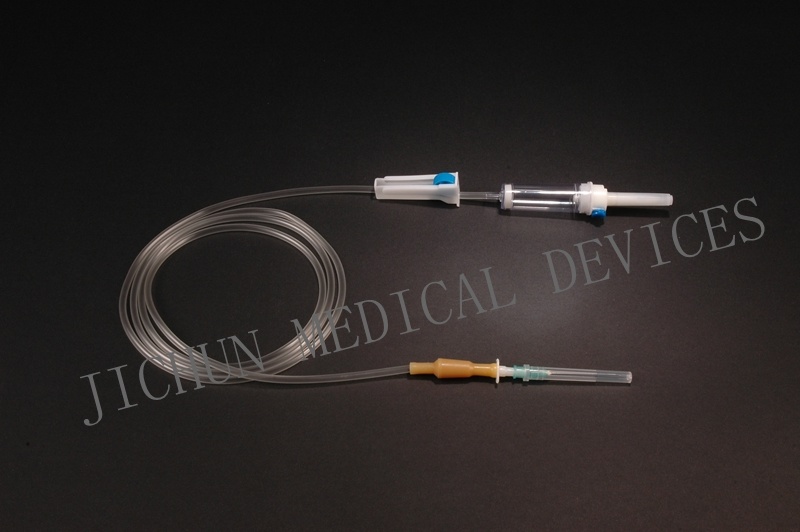
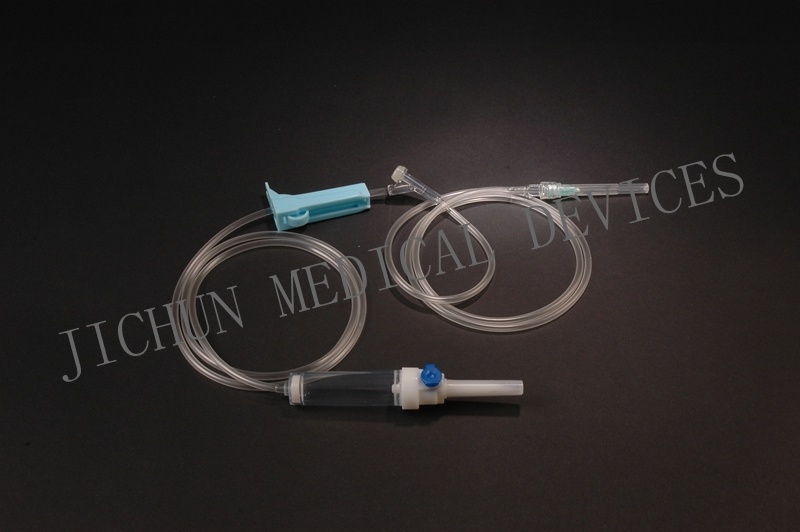
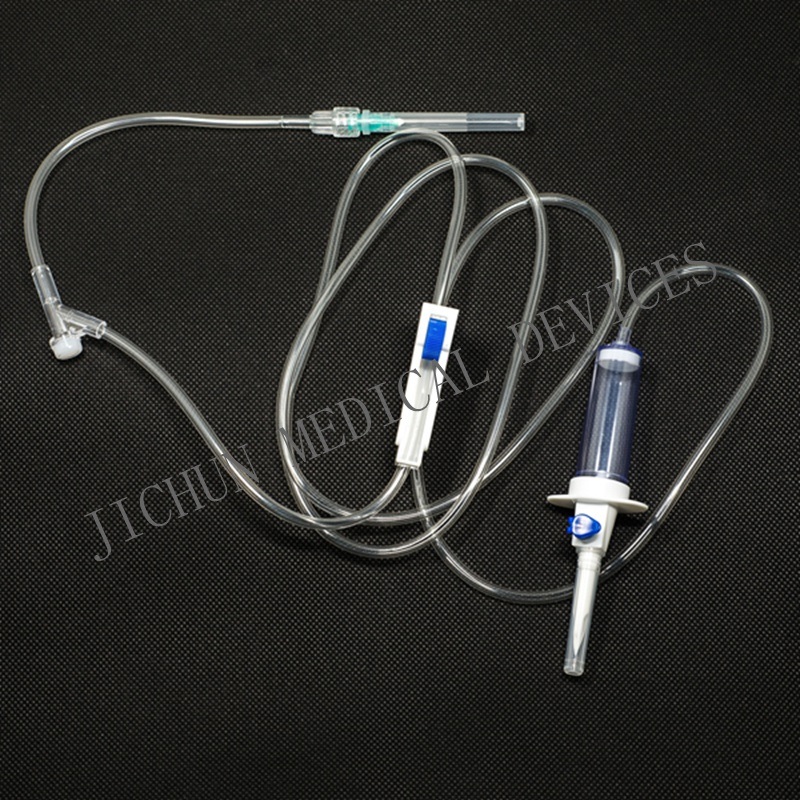 FEATURES1. Piercing device--Made of medical grade white ABS, with or without air Inlet, 3 or 4 holes size according to ISO8536-4 standard.
FEATURES1. Piercing device--Made of medical grade white ABS, with or without air Inlet, 3 or 4 holes size according to ISO8536-4 standard.
2. Protective cap--Made of PP or PE.
3. Drip chamber--Made of soft PVC, size according to ISO8536-4 Standards, 4.5cm--7cm in length.
4. Fluid filter--Support is made of ABS or PE, nylon net, mesh size: 15 micron.
5. Air inlet--Cap is made of red, white, or blue PP
6. Water-repellent filter-PP support, hydrophobic filtering paper, made of Acrylic resin and nylon.
7. Flow regulator--Made of PP or ABS, normal shape, small size or horned Shape, large size.
8. Injection site--Latex cone, rubber cone, synthetic rubber cone, Y site- Made of hard transparent PVC+injection site made of natural or synthetic Rubber.
9. Soft tubing--Made of soft medical grade PVC, diameter 3X4mm, 150cm in length, or adjust according to requirements...
10. Terminal connector: Made of PE, PVC, luer lock or luer slip.
11. Components assembly: By gluing with solvent cyclohexanoneSPECIFICATIONSThey are suited to hard containers and soft bags( without air inlet type)
Sterile, non-toxic, pyrogen free, sterilized by EOG.PACKINGÂ 1PC/blister package 20PCS/MID. PE bag, 20 bags/CTN carton size changes according to the specific volume of products. (volume is up to Chamber size, tube length, different components)
1PC/polybag (HDPE, LDPE, compound film), 20PCS/MID. PE bag, 20bags/CTN, carton size changes according to the specific volume of Products.
Remark: You can also give us your specific requirements to confirm the products you need finally.
| infusion set |
components |
MOQ |
| with different models |
can be OEM requirement |
100,000pcs |
The company mainly deals with the development, production and marketing of the medical polymer medical devices for single use. The production capacity of the main products is: 1, 000 million sets of injection needles per year, 600 million sets of syringes per year and 100 million sets of infusion devices per year. The company is with beautiful surroundings, advanced equipments, well-equipped facilities, and all kinds of advanced product measuring instruments and scientific test methods. The "Jichun" brand products produced in the company sell well worldwide, to Europe, America and Southeast Asia. Customers have high opinion of the quality and the service, with the cooperating partners and sales volum continuously increasing.
The company has a period of more than twenty years development history, with the distinctive human and geographic advantages that Changzhou is the old empire city and the location in Shanghai and Nanjing special economic zone, develops its own specialties in the aspects of injection molding, automatic assembly and packaging. The company has engaged senior top management, recruited lots of technicians, and established a complete set of quality management system. According to the requirements of ISO 13485: 2003/YY/T0287-2003 Medical devices_Quality management system_Requirements for Regulatory Purposes, Medical devices good manufacturing practice (try out), Medical devices good manufacturing practice Sterile medical devices implementation principles and inspection and evaluation standards (try out), US21 CFR Part 820 Medical devices current good manufacturing practice (cGMP), RDC59 Medical devices good manufacturing practice (GMP) and MDD 93/42/EEC Medical devices directive, assure the implementation and operation of the quality management system in the company.
In 2001 the company was appraised "credible production of quality labeled enterprise" by Chinese Association for Medical Devices Industry, Chinese Nursing Association and Chinese Fundation for Customer Protection. In 2002 the company passed ISO9002 and CE Certification, and in 2004, passed the ISO9001/ISO13485 and CE Certification. We have pioneered a developing road dominant by the precise manufacture of medical devices for single use.
In the new century, Jichun people will base on the market, look to the future, keep striving and forge ahead. We will seek the development of the company by the innovation of the concept, technology and system, to contribute to the human health.Â
PRODUCT SPECIFICATION
| Type |
infusion set |
| Material |
PVC |
| Certification |
CE, SGS ,ISO13485 |
| Sterilization |
Ethylene Oxide |
| Quality Guarantee Period |
3 years |
| Package |
PE or Blister |
| Specification |
CE , ISO , GMPÂ |
| Tube material |
PVC/Non- PVCÂ |
| Feature |
Disposable |
| Needle |
with and without needle |
| stype |
Luer Slip or Luer lock |
| Tube length |
1.5m or OEMÂ |
| Components |
Plastic Spike |
| Air vent |
With / without air vent |
| Filter |
with / without filter |





 FEATURES1. Piercing device--Made of medical grade white ABS, with or without air Inlet, 3 or 4 holes size according to ISO8536-4 standard.
FEATURES1. Piercing device--Made of medical grade white ABS, with or without air Inlet, 3 or 4 holes size according to ISO8536-4 standard.
2. Protective cap--Made of PP or PE.
3. Drip chamber--Made of soft PVC, size according to ISO8536-4 Standards, 4.5cm--7cm in length.
4. Fluid filter--Support is made of ABS or PE, nylon net, mesh size: 15 micron.
5. Air inlet--Cap is made of red, white, or blue PP
6. Water-repellent filter-PP support, hydrophobic filtering paper, made of Acrylic resin and nylon.
7. Flow regulator--Made of PP or ABS, normal shape, small size or horned Shape, large size.
8. Injection site--Latex cone, rubber cone, synthetic rubber cone, Y site- Made of hard transparent PVC+injection site made of natural or synthetic Rubber.
9. Soft tubing--Made of soft medical grade PVC, diameter 3X4mm, 150cm in length, or adjust according to requirements...
10. Terminal connector: Made of PE, PVC, luer lock or luer slip.
11. Components assembly: By gluing with solvent cyclohexanoneSPECIFICATIONSThey are suited to hard containers and soft bags( without air inlet type)
Sterile, non-toxic, pyrogen free, sterilized by EOG.PACKINGÂ 1PC/blister package 20PCS/MID. PE bag, 20 bags/CTN carton size changes according to the specific volume of products. (volume is up to Chamber size, tube length, different components)
1PC/polybag (HDPE, LDPE, compound film), 20PCS/MID. PE bag, 20bags/CTN, carton size changes according to the specific volume of Products.
Remark: You can also give us your specific requirements to confirm the products you need finally.
| infusion set |
components |
MOQ |
| with different models |
can be OEM requirement |
100,000pcs |
The company mainly deals with the development, production and marketing of the medical polymer medical devices for single use. The production capacity of the main products is: 1, 000 million sets of injection needles per year, 600 million sets of syringes per year and 100 million sets of infusion devices per year. The company is with beautiful surroundings, advanced equipments, well-equipped facilities, and all kinds of advanced product measuring instruments and scientific test methods. The "Jichun" brand products produced in the company sell well worldwide, to Europe, America and Southeast Asia. Customers have high opinion of the quality and the service, with the cooperating partners and sales volum continuously increasing.
The company has a period of more than twenty years development history, with the distinctive human and geographic advantages that Changzhou is the old empire city and the location in Shanghai and Nanjing special economic zone, develops its own specialties in the aspects of injection molding, automatic assembly and packaging. The company has engaged senior top management, recruited lots of technicians, and established a complete set of quality management system. According to the requirements of ISO 13485: 2003/YY/T0287-2003 Medical devices_Quality management system_Requirements for Regulatory Purposes, Medical devices good manufacturing practice (try out), Medical devices good manufacturing practice Sterile medical devices implementation principles and inspection and evaluation standards (try out), US21 CFR Part 820 Medical devices current good manufacturing practice (cGMP), RDC59 Medical devices good manufacturing practice (GMP) and MDD 93/42/EEC Medical devices directive, assure the implementation and operation of the quality management system in the company.
In 2001 the company was appraised "credible production of quality labeled enterprise" by Chinese Association for Medical Devices Industry, Chinese Nursing Association and Chinese Fundation for Customer Protection. In 2002 the company passed ISO9002 and CE Certification, and in 2004, passed the ISO9001/ISO13485 and CE Certification. We have pioneered a developing road dominant by the precise manufacture of medical devices for single use.
In the new century, Jichun people will base on the market, look to the future, keep striving and forge ahead. We will seek the development of the company by the innovation of the concept, technology and system, to contribute to the human health.Â
Amino Acid/Vitamins
Amino Acid,Amino Vitamins,Ethyl Ester Hydrochloride
Senyuan Biological Technology Co., Ltd. , http://www.hnvitamins.com











