European pharmaceutical industry intends to revise new regulations for Chinese medicine to eliminate barriers to Chinese medicine in Europe
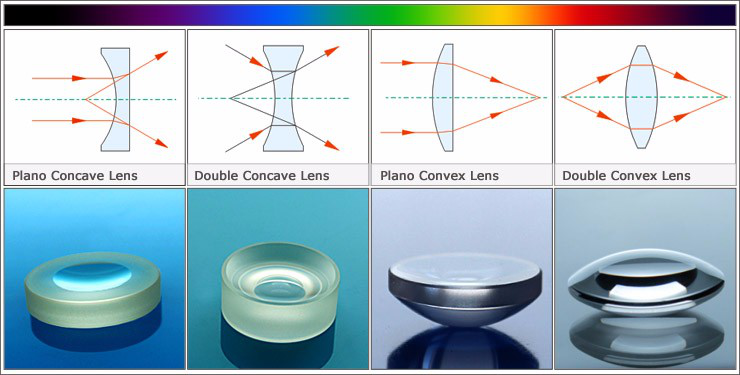
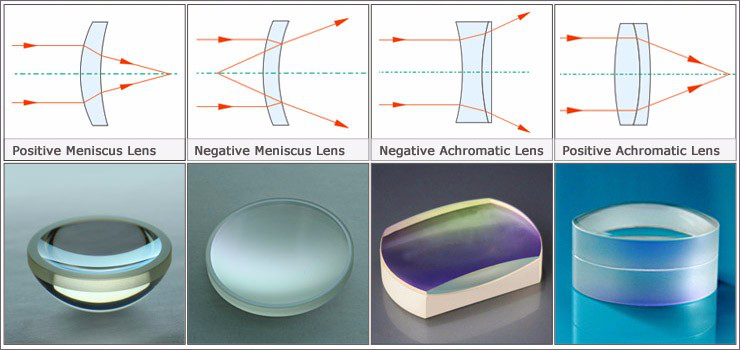
Optical Lenses are optical components designed to focus or diverge light. Optical Lenses, which may consist of a single or multiple elements, are used in a wide variety of applications from microscopy to laser processing.
Optical glass lenses have a wide variety of applications. Germanium (Ge), Silicon (Si), or Zinc Selenide (ZnSe) lenses are ideal for transmitting the Infrared (IR) spectrum, while Fused Silica is well suited for the Ultraviolet (UV).
Mini Cylindrical Lens,Precise Micro Cylindrical,Spherical Cylindrical Lenses,Spherical Ball Micro Lenses ChangChun Worldhawk Optics Co.,Ltd , https://www.worldhawk-optics.com
At the same time, it was reported that the “Traditional Plant Drugs Directive†passed by the European Commission in 2004 was caused by radical lobbying by large multinational pharmaceutical companies in order to dispel the use of traditional botanicals, especially Chinese medicines, in Europe.
The source said in an interview with reporters: “This directive is an obstacle for Chinese medicine practitioners to continue to enter the European market; if Chinese medicine companies cannot pay a high registration fee to enter the European market, multinational companies can have a greater market share,†the source said. A Chinese medicine university operating in Europe has a close relationship with European pharmaceutical regulators.
The regulation requires that from May 1 this year, any unregistered botanicals will not be sold in the EU market. The average registration of a drug takes 150,000 euros.
Initially, some multinational companies had spent heavily to lobby for a German female politician who proposed the draft legislation at the European Parliament. The source told reporters that "a key figure in the legislature who participated in this activity was telling me the origin of the decree; now, the directive has had a huge impact on the industry as a whole."
The source stated that, as is widely circulated, traditional Chinese medicines will be designated as illegal drugs due to high registration fees and complicated registration procedures. Since last year, the number of registered registrants at his Chinese University has begun to decrease. "This directive not only affects the Chinese but also affects our Europeans."
However, Thomas Brendler, chief executive of PlantaPhile Ltd., a European consultancy, believes that the purpose of the directive is to ensure the safety of consumers and remove products of doubtful quality or unreasonable health claims.
In a letter to the newspaper, Brendler wrote, “This is not a new bill, or the consequences of lobbying by a large pharmaceutical company. It merely requires that everyone follow the same guidelines. All European pharmaceutical companies, regardless of size and The size of the market influence must follow this criterion."
However, some leaders in the industry agree with the above sources. Chris Dhaenens, chairman of the Benefyt Foundation, said, “We almost agree that legislation is the result of lobbying by multinational corporations.†The foundation is based in the Netherlands and represents and protects the interests of companies and operators involved in traditional drug and formulation disputes in Europe. .
Dhaenens said that the directive initially had a clear goal of "coordinating the European herbal market", "but the results are exactly the opposite."
Dhaenens said that according to the rules, registering a product costs an average of 150,000 euros, which makes registration very difficult. "If a company has 800 products, the registration fee is equal to its total turnover and registration is impossible."
He said that registration fees vary from country to country. "This is quite undemocratic. Some countries' registration fees are much cheaper than other countries."
Dhaenens said that the directive requires traditional Chinese medicine companies to provide proof of sales in the European market for at least 15 years. At the same time, many companies find it difficult to meet the technical requirements stipulated by the European Medicines Agency, or cannot afford the required quality control (especially stability) testing costs.
Dhaenens said, "All these obstacles have prevented a Chinese company from registering."
He warned that once the directive is fully implemented on May 1, the regulators of most member states may strengthen the supervision of import companies. "These possible actions will cause difficulties and impact on European companies importing traditional Chinese medicines."
Dhaenens stated that his team is working with European institutions to change the existing legislative path - preferably by modifying existing directives, because the information and nature of the traditional Chinese medicine industry are not properly taken into account during this legislative feasibility assessment.
Wilfried Legein, president of the Belgian Traditional Chinese Medicine University, believes that if the Chinese medicine supervisors and industry alliances cooperate with their European counterparts, the chances of amending the directive still exist.
Legein said, "I don't mean that the rule is 100% not good, but if we unite to exert pressure, we have the opportunity to amend the instructions, although it will be implemented soon." Because of the issue of the law, Legegin is Belgium is hesitant to open a second traditional Chinese medicine university.
Worldhawk can supply high precision Plano Convex Lens , Double Convex Lens , Plano Concave Lens, Double Concave Lens, Meniscus Lens , Achromatic Lens and Aspheric Lens.