Analyzing the consistency evaluation of generic drugs from the number of clinical registrations of BE
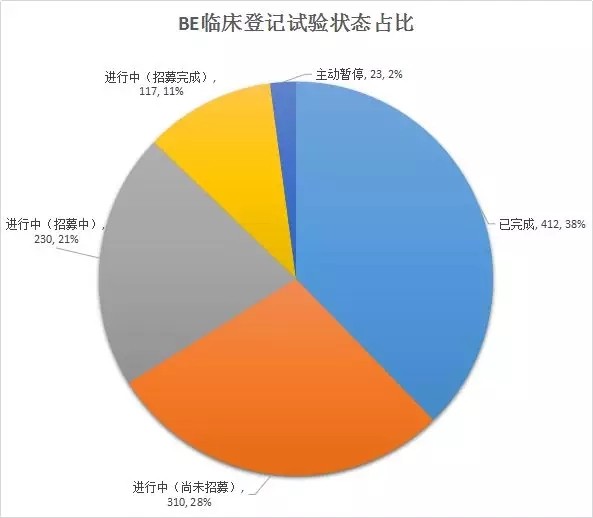
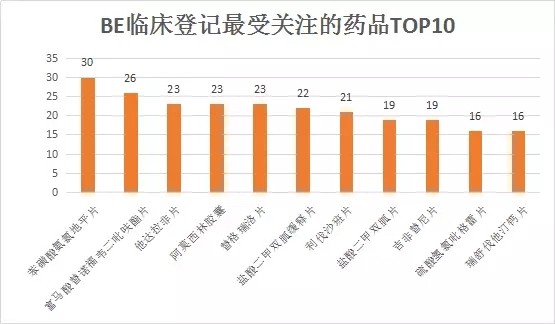
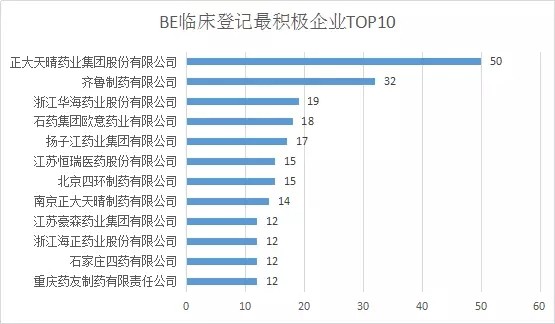
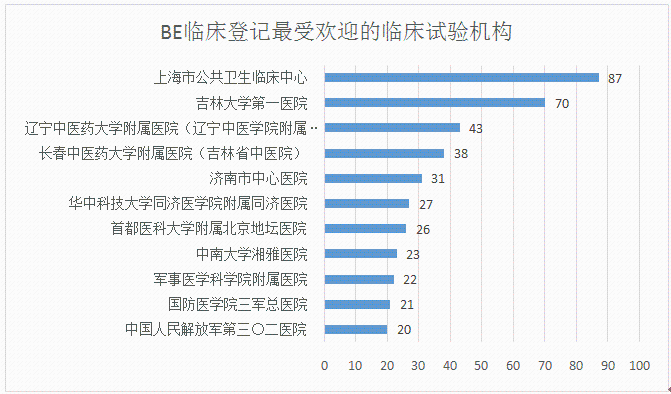
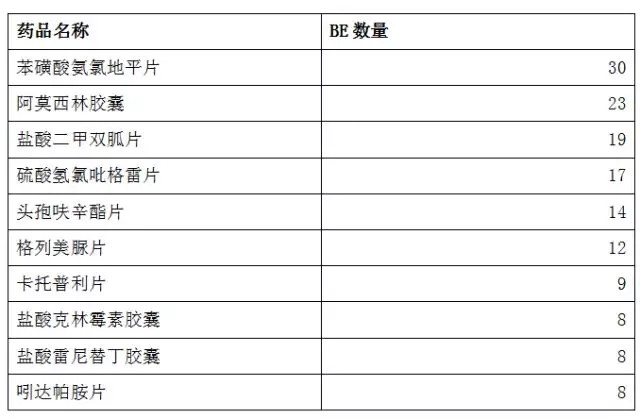
With the introduction of the “Comprehensive Evaluation of the Quality and Efficacy of Generic Drugs†policy, the focus of clinical trials has continued to increase. The data of the drug clinical trial registration platform has also increased year by year. As of April 27, 2018, the platform has obtained 8133 clinical trials of CTR, of which 5,621 were publicized. 1, BE clinical registration statistics Through the statistics from 2016 to 2018, all the "bioequivalence / bioavailability" clinical trials (not all of which are consistency assessments), as of April 27, 2018, total 1,092, containing more than 300 drugs. BE test. The following figure shows the statistics of the number of clinical registrations of BE in each month. It can be seen from the figure that with the development of the consistency evaluation of generic drugs, the number of new clinical registrations of BE has been maintained at a higher level since August 2016, reflecting from the side. The consistency assessment is accelerating. 2, BE clinical registration test status statistics In terms of test status, an analysis of 1092 BE clinical registrations revealed that 412 clinical trials have been completed, accounting for 38%; another 23 companies have actively suspended trials for some reasons, some of which are more interesting, such as registration number The reason for the suspension of CTR20171313 was that the General Administration announced the risperidone tablets of Xi'an Yangsen Original Research on December 29, 2017 as a reference preparation, so no additional research is needed. 3, BE clinical registration of the most concerned drug TOP10 According to the statistics of the drug clinical registration test of BE, the most concerned drug from 2016 to April 2018 was amlodipine besylate tablets, and the BE test reached 30; followed by tenofovir disoproxil fumarate. Ester tablets, tadalafil tablets and amoxicillin capsules. The amlodipine besylate tablets of Jiangsu Huanghe Pharmaceutical Co., Ltd. have passed the consistency evaluation; in addition, the most concerned TOP10 drugs are tenofovir disoproxil fumarate tablets, amoxicillin capsules, gefitinib Tablets, clopidogrel hydrogen sulfate tablets, rosuvastatin calcium tablets have been approved by the consistency of the enterprise. 4, BE clinical registration of the most active enterprises Overall, the number of BE clinical registrations from April 2016 to April 2018 is positively correlated with the overall strength of each company. Zhengda Tianqing Pharmaceutical Group Co., Ltd. has the largest number of registrations, with a registration number of 50. Its Nanjing Zhengda Tianqing Pharmaceutical Co., Ltd. has 14 registration numbers; followed by Qilu Pharmaceutical Co., Ltd. TOP10 enterprises are almost domestic large-scale pharmaceutical companies. It can be seen that large pharmaceutical companies pay more attention to the consistency of quality and efficacy evaluation of generic drugs, and show strong strength. 5, BE clinical registration of the most popular clinical trial institutions With the development of bioequivalence tests, clinical trial institutions have become particularly nervous, and have become the object of cooperation between CRO companies and pharmaceutical companies. On September 1, 2017, the State Food and Drug Administration and the National Health and Family Planning Commission jointly issued a notice on the human bioequivalence test for drug clinical trial institutions (No. 119 of 2017), the announcement states: According to The Drug Administration Law of the People's Republic of China, the "Measures for the Qualification of Drug Clinical Trial Institutions (Trial)", the drug regulatory authority and the health administrative department have identified 619 medical institutions with the qualifications of drug clinical trial institutions. Human bioequivalence tests can be carried out in approved clinical trials. The other contents of the announcement will not be described here. Interested friends can check the website of the Food and Drug Administration. The chart below shows the top 10 clinical trial institutions in the BE clinical registration from April 2016 to April 2018. Among them, Shanghai Public Health Clinical Center has the most popular, reaching 87 registration numbers. Careful analysis found that there were 36 clinical registration numbers in 2018. Geographically, most of the most popular clinical institutions are in the eastern and northeastern regions. 6,289 catalogue BE clinical registration analysis Presumably, readers also want to know about the 289 catalogue of drugs. According to the data, among the number of new BE clinical registrations from April 2016 to April 2018, the number of registrations belonging to the 289 catalogue was 293, accounting for 26.8% of the total 1092 registration numbers, even if the BE test was not a conformity evaluation. The ratio is still not high. On the other hand, there are only 73 drugs in the 289 catalogue of 293 registration numbers, and the overall attention is not enough. However, there are still more than 120 reference preparations for the 289 catalogue, and the company will not be in a hurry to carry out the BE test. After all, the cost of the BE test is not low and cannot be mistaken. The table below briefly lists the most popular drugs for clinical registration of the 289 catalogue of BE. No detailed analysis is done here. In general, the consistency evaluation of generic drugs is undoubtedly the hottest topic in the past three years, and the dissolution and BE test are the key to consistency evaluation. With the development of complex injection consistency evaluation, the BE test will still be highly concerned.
Kinesiology tape
Jerry tape is specialized in Anti Slip Tape for 10 years. Our Kinesiology tape is very welcomed by most customers.
Powerful and durable tape - Effective skin preparation can last for several days as the latest technology. Our synthetic, moisture-absorbing and breathable fabrics provide maximum waterproof, sweat-proof and moisture-proof properties. Pre-tangential roundness of motion tape helps to avoid wear and tear.
2. Features of Jerry tape Kinesiology Tape
1).100% high quality cotton stretch fabric
6)Single shrink package or OEM package
3. Applications of jerry tape Kunesiology Tape
1).Protective muscle
Kinesiology,Tape,Colorful,Cotton,Sport Tape,athletic,safety premium Kunshan Jieyudeng Intelligent Technology Co., Ltd. , https://www.jerrytapes.com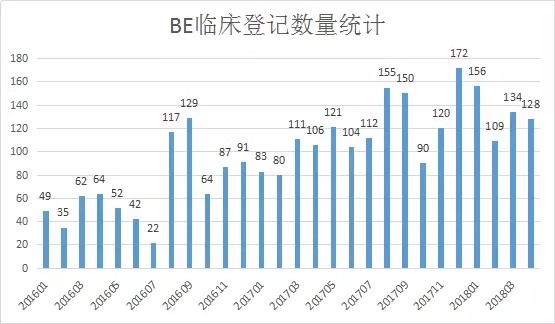
Best analgesic tape - the most advanced sports Kinesiology tape, made of non-latex 95% cotton 5% elastic man-made fiber, has longer service life than other brands. Perfect pain relief for muscles, tibial splints, knees and shoulders. Lightweight, waterproof, latex-free, comfortable, providing support and flexibility, including safe reflective printing and suitcases.



2).Medical environmental protection glue
3).Temperature range 0° to 60°.
4).Widly weclomed size 50mmx5m,or custom size
5).Skined,Black,Blue etc color,printed customed design
2).Supporting Tape for your muscle
3).Effective to releive - fatigue at leg or thigh etc
4).Knee Pain(Gonalgia), Low Back Pain(LBP) and Shoulder Pain(Omalgia)
5).Effective to prevent Angle Sprain